HCY disposable virus transport media was approved by FDA 510K
5778Recently, the disposable virus transport media developed and produced by Huachenyang has obtained the FDA 510K certification in the United States
View detailsSearch the whole station Lab Supply

On June 26th, Huachenyang’s general specimen collection kit (virus transport media) was officially certified by UKCA, indicating that Huachenyang’s virus transport media meets the latest market access and conformity assessment requirements in the UK.
This also means that the quality and safety of Huachenyang’s products are officially certified and can be legally sold and used in the United Kingdom and countries and regions that recognize the UKCA registration, marking another important step for Huachenyang to enter the European market.
UKCA (United Kingdom Conformity Assessed) is a new product certification introduced after the UK’s exit from the European Union. Electrical and electronic products entering the UK market need to pass the standard tests under the UK regulations and obtain the UKCA certificate, and the products are marked with the UKCA logo to replace the EU CE marking in the UK market. Specific products such as automotive, aerospace, pharmaceutical products, medical devices, chemicals, and articles subject to national regulations (non-harmonized articles) will not be able to continue to use the EU norms and conformity marking when they are re-sold in the UK market after Brexit.
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.
Recently, the disposable virus transport media developed and produced by Huachenyang has obtained the FDA 510K certification in the United States
View detailsThe DNA Collection Kit includes sampling swabs and sample preservation tubes for improved usability.
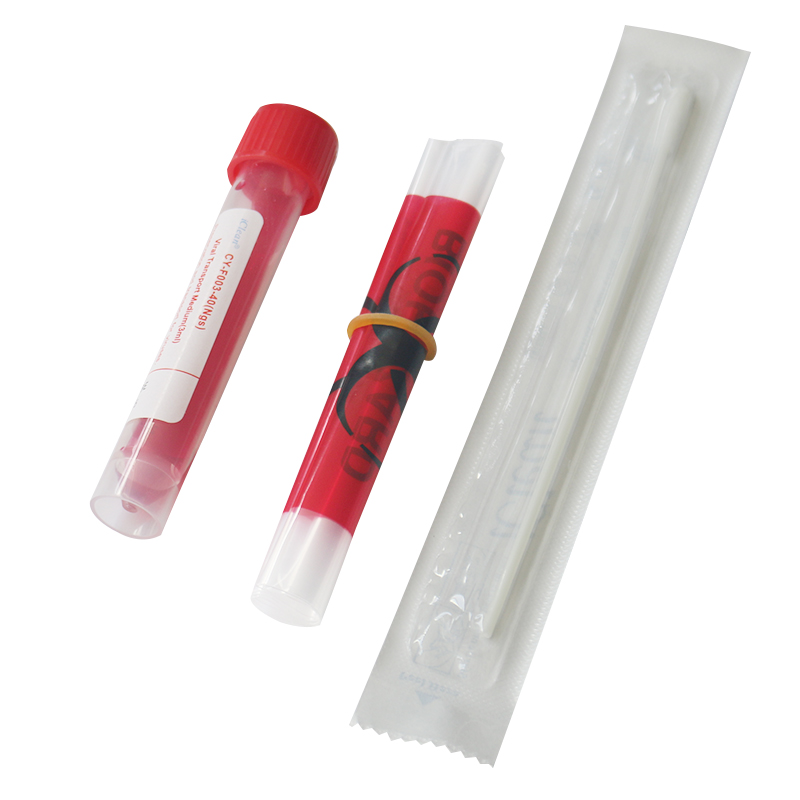
View detailsThe red liquid in the virus transport medium is called virus preservation fluid.
View detailsThe viral transport medium(VTM) has only one mission - to keep the genetic material in the test sample intact.
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy