What happens when you touch the liquid in the virus transport medium?
6224The red liquid in the virus transport medium is called virus preservation fluid.
View detailsSearch the whole station Lab Supply
As the United States navigates the peak of the 2025-2026 influenza season, public health systems are facing significant pressure. The Centers for Disease Control and Prevention (CDC) estimates that by late December, the virus had already caused at least 7.5 million illnesses, 81,000 hospitalizations, and 3,100 deaths. This surge is largely driven by a dominant and challenging strain: the Influenza A(H3N2) subclade K, often dubbed the “super flu”. With 32 jurisdictions reporting “high” or “very high” levels of respiratory illness activity, the demand for accessible, efficient, and reliable diagnostic solutions has never been more critical.
The current landscape presents a dual challenge: a highly transmissible virus and strained healthcare resources. The subclade K strain, which accounts for nearly 92% of analyzed flu samples, is notable for its ability to partially evade prior immunity, leading to widespread infection. Concurrently, emergency departments are experiencing increased visits, and vaccination rates—while a vital tool for preventing severe outcomes—remain lower than desired, with only about 130 million doses administered this season.
In this environment, traditional testing pathways—requiring sick individuals to travel to clinics, urgent care centers, or hospitals—pose a significant burden. They increase the risk of exposure for vulnerable patients and healthcare workers and can delay diagnosis and treatment. Timely antiviral treatment for influenza is most effective when started within one to two days of symptom onset, making rapid and convenient testing a key component of effective management.
This is where telemedicine-guided diagnostic solutions and at-home self-sampling emerge as transformative tools for modern healthcare delivery. The pandemic era catalyzed a massive shift toward contactless health care services, proving that remote diagnostic models are not only feasible but also highly satisfactory for users. A 2024 feasibility study published in JMIR Formative Research demonstrated that a comprehensive telemedicine approach for SARS-CoV-2 self-sampling achieved a 95% sample receipt rate and a 76% successful return rate for diagnostics. Users reported high satisfaction with the model’s ease of use and interface. This validated framework provides a ready-made blueprint for addressing the current influenza crisis.
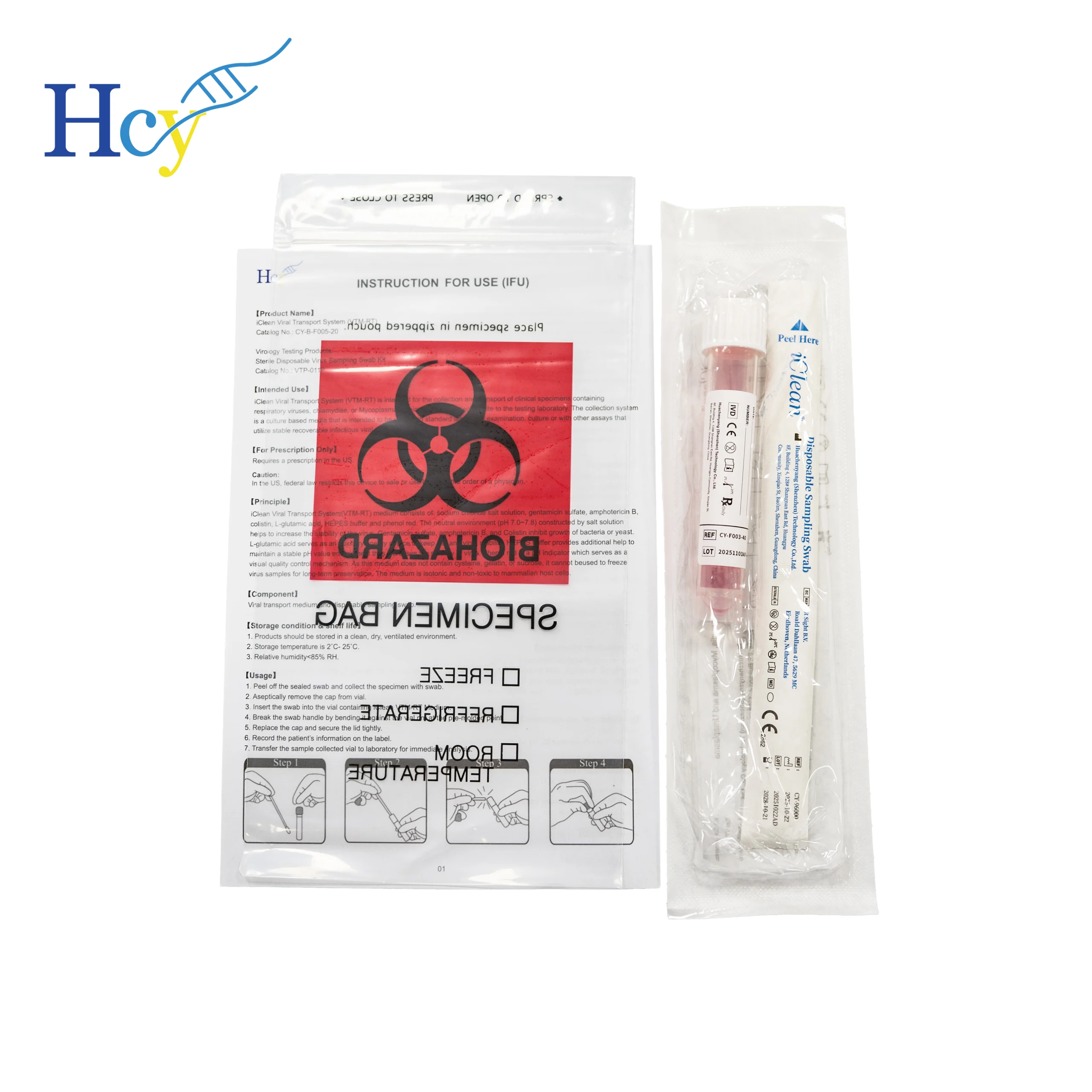
At the heart of any reliable remote testing program is a robust and user-centric sample collection system. This is the domain of the Viral Transport Medium (VTM) kit. For companies like Huachenyang Technology Co., Ltd., specializing in diagnostic specimen collection, this moment represents a critical opportunity to support public health. Our VTM sampling kits are engineered to bridge the gap between the patient’s home and the clinical laboratory with uncompromising integrity.
The core function of a high-quality VTM is twofold: preservation and inactivation. Specimens must remain viable for accurate polymerase chain reaction (PCR) testing, the gold standard for sensitivity and specificity in viral detection. Concurrently, ensuring the virus is inactivated within the medium is crucial for safe transport and handling. Research cited by the National Institute of Standards and Technology (NIST) confirms that certain VTM formulations can completely inactivate high-titre SARS-CoV-2 in as little as two minutes, rendering samples non-infectious and safe for point-of-care processing or transport. This dual capability makes VTM kits indispensable for decentralized testing models.
Our VTM sampling kits are designed with the end-user—often an anxious individual feeling unwell—in mind. Each kit contains clear, pictogram-based instructions and all necessary components: sterile swabs for oropharyngeal or midturbinate sampling, a leak-proof tube containing the optimized VTM, and secure packaging compliant with transport regulations (e.g., UN 3373). The goal is to make the self-collection process intuitive, thereby minimizing user error and ensuring the sample collected at home is as reliable as one collected by a healthcare professional.
The integration pathway is straightforward and leverages existing digital health infrastructure. A patient experiencing flu-like symptoms initiates a consultation via a telehealth platform. If testing is indicated, a Huachenyang VTM kit is dispatched directly to their home. Upon arrival, the patient follows the guided instructions to collect their sample, places it in the pre-labeled transport box, and returns it via mail or a dedicated courier service. The sample is then processed at a partnered clinical laboratory, and results are communicated securely through the platform, often with follow-up care guidance.
This model delivers profound benefits:
The evidence for this shift is compelling. Beyond the SARS-CoV-2 study, VTM-based kits have been successfully used in protocols for a range of respiratory virus detection and genomic surveillance studies worldwide. The current U.S. flu season, marked by the rapid spread of a distinct subclade, underscores the urgent need for agile, scalable, and patient-friendly testing solutions that can keep pace with viral evolution.
For healthcare providers, telehealth companies, and corporate wellness programs looking to offer a superior standard of care during this severe flu season and beyond, partnering with a trusted supplier of medical specimen collection products is essential. Huachenyang Technology’s VTM kits represent more than just a product; they are a critical enabler of a more resilient, responsive, and patient-centered diagnostic ecosystem.
The “super flu” season is a stark reminder that our approach to infectious disease management must evolve. By embracing the proven model of telemedicine-based diagnostics and supporting it with reliable, professional-grade at-home self-sampling kits, we can build a frontline defense that meets people where they are—safely, conveniently, and effectively.
The red liquid in the virus transport medium is called virus preservation fluid.
View detailsVTM allows the safe transfer of viruses, chlamydiae, and mycoplasma for further research and analytics. The special formulation ensures the best possible recovery of the samples and the addi...
View detailsOn June 26th, Huachenyang's general specimen collection kit (virus transport media) was officially certified by UKCA
View detailsThe full name of VTM is Virus Transport Medium, which is mainly used for collecting, transporting and storing biological samples.
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy