The correct usage method of flocked swabs
1185Wash hands and wear gloves before use, tear open the individual packaging and remove the swab to avoid touching the head of the swab with your hands.
View detailsSearch the whole station Lab Supply
Huachenyang single-use sampling swab has recently been registered by the Medicines and Healthcare products Regulatory Agency(MHRA) in the UK. available for sale in the UK and nations that accept MHRA registration.
MHRA is the executive government agency under the UK Department of Health and Social Care. It is responsible for ensuring the efficacy and safety of medications and medical devices.


The EU CE certification will no longer be recognized after June 30, 2023, as per the Brexit agreement. To supply medical devices in the UK, a company must have a UK Responsible Person (equivalent to an EU Authorized Representative) and be registered with the UK Responsible Person with the MHRA.
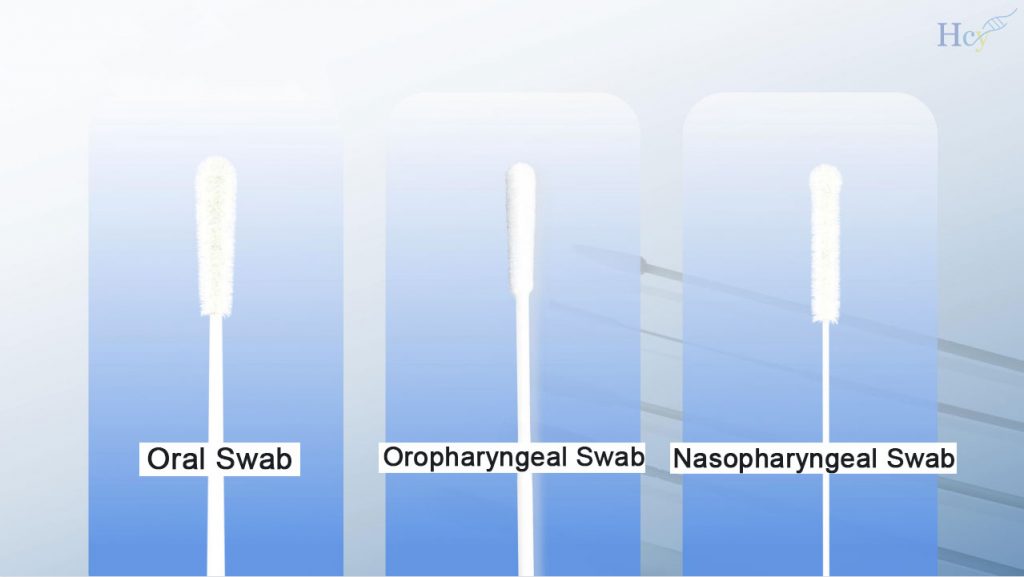
Fine flocked fibers are bonded to create a velvety brush using cutting-edge jet-implanted nylon fiber technology. Maximizing the sampling efficiency. Nylon fibers adhere vertically and uniformly to the swab head surface which considerably enhancing the efficiency of sample collection and release to enhancing detection precision.
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.
Wash hands and wear gloves before use, tear open the individual packaging and remove the swab to avoid touching the head of the swab with your hands.
View detailsDisposable sterile swabs have become an indispensable tool in various fields, particularly in the medical industry.
View detailsThe oropharyngeal flocked swab can quickly complete sample collection and store samples in the tube, which facilitates sample transportation.
View detailsAlthough the sample swab resembles a cotton swab, it is not one.
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy