Video: How to use iClean COVID-19 Antigen Rapid Test Kit?
3767How to use iClean COVID-19 Antigen Rapid Test Kit? Here is a video tutorial.
View detailsSearch the whole station Lab Supply
The European Health Security Committee (HSC) has created a whitelist of COVID-19 rapid antigen test products to prevent the inconsistent quality that are acknowledged by member states known as the “HSC Common List,” and its test results will be acknowledged in the 27 member states of the EU. Before a product is included to the Common list, manufacturers must offer more clinical verification evidence to demonstrate the product’s effectiveness.

HCY COVID-19 Ag Rapid Test Kit is included in the latest EU Common List. Previously, the product also obtained EU CE2934 certification and Thailand TFDA certification.
From June 1, 2022,The EU common list of COVID-19 antigen tests has been split up in two categories.
Category A: Antigen tests for which their performance has been evaluated through prospective clinical field studies and that meet the criteria agreed.
Category B: Antigen tests for which their performance has been evaluated through retrospective in vitro studies and that meet the criteria agreed.
EU Member States are strongly encouraged to use, in particular, antigen tests included under
Category A of the EU common list for the issuance of EU Digital COVID certificates.
HCY COVID-19 Ag Rapid Test Kit has passed prospective clinical field studies in German and Greek laboratories and is therefore classified as Category A.

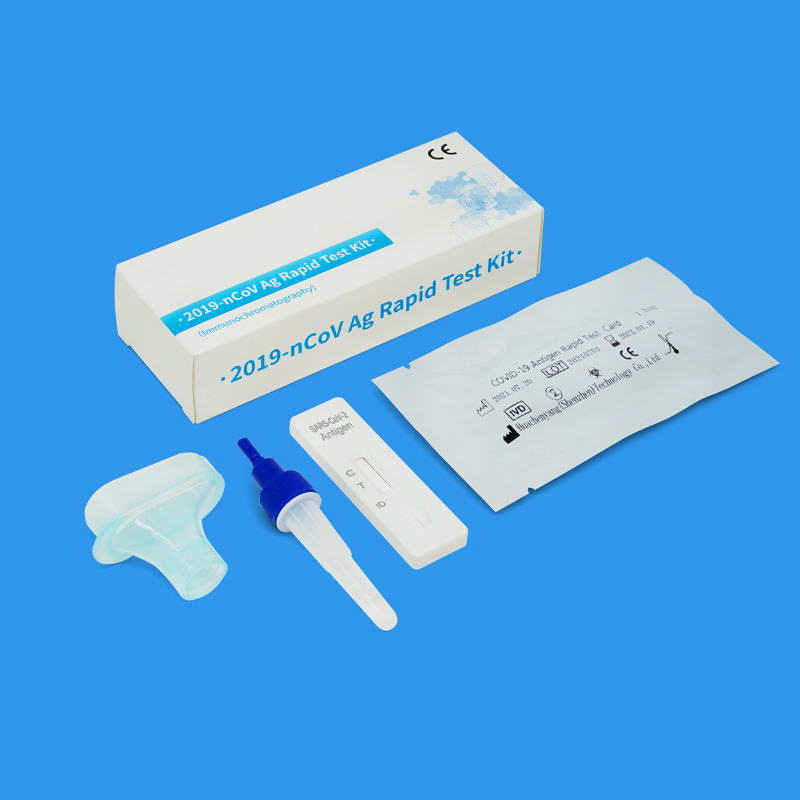
Huachenyang COVID-19 Ag Rapid Test Kit have great reputation in EU for its fast, convenient and accurate advantages.It is recognized as quick and reliable detection techniques.
Winter is coming, the pandemic is not going away, and the several varieties are raging outside. One of the keys to halting the pandemic from spreading is swift and efficient testing.
We have developed COVID-19 Ag Rapid Test Kit Professional Version and the Self-Test Version, each version could be customized in order to satisfy diverse usage scenarios.
“HCY, Health care for you” is our forever mission. We dedicate to offering safe & reliable products and medical services with our global creditable partners. HCY has already supplied to WHO, MAYO clinic, MGI, DDC, Yale University, Qorvo, Quanterix, Thomas Scientific, SD biosensor, Cardinal Health, Cleveland Clinic, Mars Petcare & LumiraDx, etc. in the past years.
How to use iClean COVID-19 Antigen Rapid Test Kit? Here is a video tutorial.
View detailsThe COVID-19 Ag Rapid Test Kit uses the double antibody sandwich method to legally detect the novel coronavirus antigen in nasopharyngeal swabs.
View detailsThe COVID-19 Antigen Test is a method for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigens from the saliva.
View detailsInformation on types of COVID-19 tests and how they work. Rapid antigen point-of-care tests are performed by health practitioners, or trained persons under their supervision. This ensures an ad...
View detailsWe value your privacy We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.
Our Privacy Policy